How advanced is Nanotechnology today?
Nanotechnology is in it's infancy but there are a number of concentrated efforts and surprising advancements. This section describes the current efforts and highlights some of the most notable discoveries and products in contemporary Nanotechnology.
"Wet" Nanotech applications

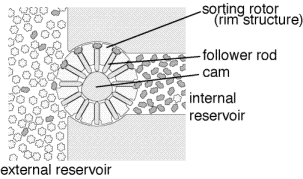
This is an example of a pump for wet nanotech, it can
deliver drugs or other substance at the cellular level.
Wet nanotech applications are already in
use manipulating DNA and protiens. Proteins are molecular machines that
routinely manipulate individual atoms. Proteins have unique physical structure
and functionality. Protein engineers can now synthesize all 20 natural
proteins and even design synthetic ones with novel properties. Scientists
are attempting to catalog the functions of proteins, how they fold, and
discover properties of synthetic proteins. Chemists are also synthesizing
larger and more complicated molecules that preform very complex physical
tasks and even self replicate. Since this is so colsely related to biotechnology
only a brief description of "wet" nanotechnology is included.
"Dry" Nanotech applications
Buckyballs and Buckytubes
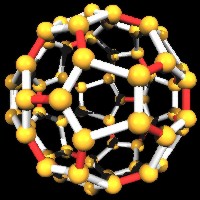
A good color pic of a bucky ball.
Buckyballs are microscopic balls made of carbon that are formed from a graphene sheet and look like little soccer balls. The incredible strength of these balls, coupled with their natural tendency to self-assemble, inspired scientists to develop the Buckytube, the strongest possible materal made with known matter. The Buckytube could be the best example of current success in nanotechnology. Read on to discover the amazing properties and potential of the Buckytube.
Buckyballs were discovered in 1985 at Rice University in Houston, Texas. Dr. Richard Smalley discovered a unique property of carbon when it was heated. "When you vaporize carbon, mix it with an inert gas and then let it condense slowly, it turns out that if you don't let it become too cold too quickly there is a wonderful self-assembly process where the carbon atoms hook in together to make graphene sheets that start to curl around, their incentive being to get rid of the dangling bonds on the edge. It turns out that with amazingly high probability it will close into a closed geodesic dome composed of some number of hexagons and 12 pentagons."
This process developed a new type of
carbon chain that is extremely strong and stable and closed on both ends,
it was named C60. Dr. Smalley explains why C60 is so powerful. "C60
is special because of all the structures made of pentagons and hexagons
that can curl around and close, there is only one that can do it so smoothly
that every atom has the same curvature as every other. This is a consequence
of mathematics. Sixty is the most factorable of all integers. That's why
the Babylonians used it as the base of their number system, that's why
we still divide circles into 360 degrees, and why we have 60 minutes in
an hour and 60 seconds in a minute. For reasons that so far seem obscure
but probably are connected somehow to its high factorability, sixty is
also the maximum finite number of ways you can rotate an object around
a central point in 3 dimensional space so that when you finish rotating
it looks exactly the same as before. Such an object has the symmetry of
the icosahedron, the highest finite point group, which has 60 proper rotational
symmetry elements. "
What are the properties of Buckytubes
and how can they be produced? Fullerene tubes expert Richard Smalley
observes the Buckytube's preferred diameter is 1.1 nanometer (1.1 billionths
of a meter) although smaller and larger multi-nested-tubes
exist. Tubes form (instead of Buckyballs) when graphite
spiked with 1% catalyst from the nickel, cobalt and iron group is heated
above 3000 C and the resulting vapor at a 1200 C. The metal atoms react
with dangling bonds at the end of a tube and favor tube production by arriving
carbon vapor over hemispherical capping. If you do not use the catalyst
you get buckyballs.
Unlike a diamond's three-dimensional crystal lattice, Buckytubes are one atom thick sheets curled to make a hollow tube in which all four of a carbon's bond potentials are used more or less in enforcing a one dimensional structure that runs the length of the tube. Every bond is involved in making the tube strong lengthwise. This allows the Buckytube to surpass even the diamond in strength.
Dr.Smalley does an excellent job of
visualizing the size of a Buckytube. " A single walled carbon Buckytube
molecule looks like a tube of chicken wire (hexagons) capped by an arrangement
of hexagons and pentagons and is generally around 1.1 or so
nanometers in diameter. By comparison, the red blood
cell is a giant at 5,000 nanometers wide. If a Buckytube were blown up
to a foot in diameter, then a red blood cell, standing
on end would be a cliff nearly a mile high. A Lincoln head penny standing
on end next to the blood cell would vault out of the
atmosphere to a height of some 3,300 miles. Place another such penny in
the same hemisphere on the other side of the Earth, and
to a distant space traveler the planet would resemble a Disney
character. Your average single wall tube is really small!"
Buckytubes are the strongest possible material that can currently be made with known matter (not considering some theoretical possibilities of carbon nitride). The strength of a Buckytube is predicted to be somewhere between 1.5 and 3 times that of a diamond fiber, or 100 times as strong as steel at only one quarter the weight. The manufacturing of nanometer sized graphitic tubes will open the eyes and the economies of society and industry to the unprecedented power of nanometer scale technology. This one self assembling molecule will give us a glimpse of an incredibly different future, a future of super performance materials.
Dr. Richard Smalley of Rice University is a Nobel prize recipient for co-discovery of Buckyballs and is busy in the lab working on the development of tubes of unlimited length. We are on the threshold of obtaining an incredible new material... that is a sign of things to come.
These tubes are the self assembling
"ropes" and "rods" of the nanometer realm, lending themselves to applications
such as transferring power between molecular machines. Shorter, stiff multi-walled
tubes might be used for rod logic computers or frames with which to hang
components of nanomachines.
The Buckytube can be used to move
electricity with great effeciency. Buckytube cables are predicted
to be 50-100 times as conductive as copper. If the tubes could be made
inexpensively, a revolution in power transmission efficiency would be at
hand. This could help make computers and communications much faster and
more efficient. This technology could also aid in superconductivity research
efforts.
Strength and conductivity are two very promising applications of Buckytubes. The performance advantages are not just small advaces, Buckytubes are up to 100 times stronger and/or more conductive than anything available today. The changes that take place could be monumental. Here is a small vision of Smalley's: "Buckytubes should make fabric with some really dazzling properties and applications like anti-abrasion coats. How do you wear out a fabric tougher than diamond? Much improved bullet proof vests and new armor may appear. Sail cloth many times superior to Kevlar may be in the works. A large four person stand up tent with poles may weigh only ounces, parachutes, less."
It is clear that there are a number
of advantages of this new form of carbon, but what are the disadvantages?
The first problem is associated with cost.C60 and simular carbon composites
(C70, C72, C74) are available commercially. They may be cheaper than they
used to be, but they are still very expensive. C60, which is the cheapest
of what's available is still about 5 times more expensive than gold. This
high cost is probably the reason that there is not a marketable product
using this technology available today. Another disadvantage is manipulation
of buckytubes. How would you cut Buckycloth? Standard metal scissors
would not work. Buckyrope may prove to be a great band saw for the hardest
metals and stone. Since you cannot break these carbon chemical bonds with
cutting devices made of weaker bonds, lasers producing temperatures above
3,000 C just might be the best candidate. You have to blast a Buckytube
with enough concentrated energy to over come the short, super strong
bonds. Fortunately, such industrial laser cutting tables have existed for
quite awhile.
Dr. Smalley is continuing his research
in this field he helped pioneer and his home page is a great place to learn
more about the potential of carbon and the graphene sheet. He is
already capable of producing tubes 100,000 times as long as the diameter
of the tube. His homepage suggests the possibility of unlimited fiber
length by the end of the decade. This achievement would usher in the age
of Fullerene super materials.
"Computational" Nanotech applications
Quantum Dots
Quantum dots are one of the first currently active nano-scale computational devices. A quantum dot can be thought of as box that holds or releases electrons. They hold a certain number of electrons that can be manipulated by adjusting nearby electric fields.
Dr.Craig Lent of Notre Dame suggests, that arraging these dots into cells to create a sort of binary logic. Given a four quantum dot cell, Lent proposes, "We arrange that two electrons reside in each cell. They may hop (tunnel) from dot to dot within the cell to find a favorable, low-energy configuration. Of course, because electrons are charged, they repel each other, so the two electrons will not both choose to sit in the same dot within the 4-dot cell. In fact, they will choose to occupy two opposite corners of the cell, since this gives them their maximum possible separation. The electrons would be equally happy in ... two configurations. This is nice, because it gives us two possible "polarization" states for a cell, which we can associate with a binary zero and one."
"We can use a voltage on an external
gate to force a cell into one of its two a priori equally good alternative
states. This is a signal, analogous to a high or low voltage at a point
within a conventional integrated circuit. A conventional integrated circuit
uses two tools to transmit a voltage signal over long distances: an aluminum
or silicon wire (to carry signals from place to place) and a transistor
used as an amplifier (to refresh weakened signals so that they may travel
farther without corruption). In the
quantum dot circuits proposed by Lent, both of these
tasks are accomplished simply by arranging as many cells as necessary in
a row. If two cells are placed next to each other, and the polarization
of first is fixed, the second will match its own polarization to that of
the first:" (Goldhaber-Gordon, MIT)
The advantage here as with all computational nanotechnology initiatives is size. If the distance between dots in a cell or array is 20nm an adder could fit in an area of as little as 1 micron^2. Currently computer industry estimates are that by 2010 one transistor will be about 1 micron^2 and it takes forty transistors to make an adder. Compueter processor sizes could be microscopic. This is quite a step forward and not unreasonable to build with todays methods of chip manufacture.
The drawbacks here are great but not insurmountable. The first problem is that the architechture for quantum cells is quite different from ordinary IC's. With current components there are two inputs and one output. With quantum cells there are three inputs. Dr. Lent explains that this is not a problem. He says that the inputs simply use the majority input to give the desired output. For example inputs 1 and 2 will decide an outcome that will be compared with input 3 for a final output. He suggests that by fixing one of the first inputs the will work like a binary device.
The biggest drawback to this architecture is ambient thermal energy. The ambient thermal energy at room temperature is twenty times greater than the energy needed to create errors in quantum dots seperated by the proposed 20nm distance. The problem here is that thermal energy could confuse the electrons in the dots by changing the "lowest energy state". This means that the electrons may not always jump to the predicted site and thus result in errors. Different directions are being studied to remedy this problem but no solutions are available yet.